Abstract
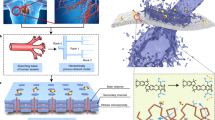
Nuclear power could continue to be a reliable and carbon-free energy source at least from a near-term perspective. In addition to the safety issues, another risk that may threaten the sustainability of this technology is the uranium supply disruption. As opposed to the land-based deposits, the ocean contains 1,000 times more uranium reserves and provides a more abundant resource for uranium. However, due to the very low concentration and presence of many other metal ions as well as the accumulation of microorganisms, the development of uranium extraction technology faces enormous challenges. Here we report a bifunctional polymeric peptide hydrogel that shows not only strong affinity to and selectivity for uranium in seawater but also remarkable resistance against biofouling. Detailed characterizations reveal that the amino acid in this peptide material serves as the binding ligand, and uranyl is exclusively bound to the oxygen atoms. Benefiting from its broad-spectrum antimicrobial activity, the present polymeric adsorbent can inhibit the growth of approximately 99% of marine microorganisms. Measurements in natural seawater show that this peptide material delivers an impressive extraction capacity of 7.12 mg g−1 and can be reused. This work opens a new direction for the design of low-cost and sustainable materials for obtaining nuclear fuel.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available in the paper and its Supplementary Information.
References
Chu, S. & Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012).
Peng, W. et al. Managing China’s coal power plants to address multiple environmental objectives. Nat. Sustain. 1, 693–701 (2018).
DeRolph, C. R., McManamay, R. A., Morton, A. M. & Nair, S. S. City energysheds and renewable energy in the United States. Nat. Sustain. 2, 412–420 (2019).
Sholl, D. S. & Lively, R. P. Seven chemical separations to change the world. Nature 532, 435–437 (2016).
Dye, S. T. & Guillian, E. H. Estimating terrestrial uranium and thorium by antineutrino flux measurements. Proc. Natl Acad. Sci. USA 105, 44–47 (2008).
Abney, C. W., Mayes, R. T., Saito, T. & Dai, S. Materials for the recovery of uranium from seawater. Chem. Rev. 117, 13935–14013 (2017).
Davies, R. V., Kennedy, J., Mcilroy, R. W., Spence, R. & Hill, K. M. Extraction of uranium from sea water. Nature 203, 1110–1115 (1964).
Xu, X. et al. 3D hierarchical porous amidoxime fibers speed up uranium extraction from seawater. Energy Environ. Sci. 12, 1979–1988 (2019).
Sun, Q. et al. Covalent organic frameworks as a decorating platform for utilization and affinity enhancement of chelating sites for radionuclide sequestration. Adv. Mater. 30, 1705479 (2018).
Das, S. et al. Novel poly(imide dioxime) sorbents: development and testing for enhanced extraction of uranium from natural seawater. Chem. Eng. J. 298, 125–135 (2016).
Luo, W. et al. Engineering robust metal-phenolic net work membranes for uranium extraction from seawater. Energy Environ. Sci. 12, 607–614 (2019).
Chen, Z. et al. N, P, and S codoped graphene-like carbon nanosheets for ultrafast uranium (VI) capture with high capacity. Adv. Sci. 5, 1800235 (2018).
Liang, P. L. et al. Photocatalytic reduction of uranium(VI) by magnetic ZnFe2O4 under visible light. Appl. Catal. B 267, 118688 (2020).
Yuan, Y. et al. Molecularly imprinted porous aromatic frameworks and their composite components for selective extraction of uranium ions. Adv. Mater. 30, 1706507 (2018).
Sun, Q. et al. Bio-inspired nano-traps for uranium extraction from seawater and recovery from nuclear waste. Nat. Commun. 9, 1644 (2018).
Chong, L. et al. A half-wave rectified alternating current electrochemical method for uranium extraction from seawater. Nat. Energy 2, 17007 (2017).
Cui, W. R. et al. Regenerable and stable sp2 carbon-conjugated covalent organic frameworks for selective detection and extraction of uranium. Nat. Commun. 11, 436 (2020).
Sun, Q. et al. Spatial engineering direct cooperativity between binding sites for uranium sequestration. Adv. Sci. 7, 2001573 (2020).
Qin, Y. et al. Flexibility and intensity of global water use. Nat. Sustain. 2, 515–523 (2019).
Kou, S., Yang, Z. & Sun, F. Protein hydrogel microbeads for selective uranium mining from seawater. ACS Appl. Mater. Interfaces 9, 2035–2039 (2017).
Lejars, M., Margaillan, A. & Bressy, C. Fouling release coatings: a nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 112, 4347–4390 (2012).
Kuo, L. J. et al. Characterization and testing of amidoxime-based adsorbent materials to extract uranium from natural seawater. Ind. Eng. Chem. Res. 55, 4285–4293 (2016).
Ivanov, A. S. et al. Origin of the unusually strong and selective binding of vanadium by polyamidoximes in seawater. Nat. Commun. 8, 1560 (2017).
Brown, S. et al. Uranium adsorbent fibers prepared by atom-transfer radical polymerization (ATRP) from poly(vinyl chloride)-co-chlorinated poly(vinyl chloride) (PVC-co-CPVC) fiber. Ind. Eng. Chem. Res. 55, 4139–4148 (2016).
Gill, G. A. et al. The uranium from seawater program at the Pacific Northwest National Laboratory: overview of marine testing, adsorbent characterization, adsorbent durability, adsorbent toxicity, and deployment studies. Ind. Eng. Chem. Res. 55, 4264–4277 (2016).
Park, J. et al. Effect of biofouling on the performance of amidoxime-based polymeric uranium adsorbents. Ind. Eng. Chem. Res. 55, 4328–4338 (2016).
Sun, G. L., Reynolds, E. E. & Belcher, A. M. Using yeast to sustainably remediate and extract heavy metals from waste waters. Nat. Sustain. 3, 303–311 (2020).
Wang, X. M. et al. A 3,2-hydroxypyridinone-based decorporation agent that removes uranium from bones in vivo. Nat. Commun. 10, 2570 (2019).
Zhou, L. et al. A protein engineered to bind uranyl selectively and with femtomolar affinity. Nat. Chem. 6, 236–241 (2014).
Bai, Z. et al. Mussel-inspired anti-biofouling and robust hybrid nanocomposite hydrogel for uranium extraction from seawater. J. Hazard. Mater. 381, 120984 (2019).
Yu, Q. H. et al. A universally applicable strategy for construction of anti-biofouling adsorbents for enhanced uranium recovery from seawater. Adv. Sci. 6, 1900002 (2019).
Byers, M. F., Landsberger, S. & Schneider, E. The use of silver nanoparticles for the recovery of uranium from seawater by means of biofouling mitigation. Sustain. Energ. Fuels 2, 2303–2313 (2018).
Yuan, Y. H. et al. Ultrafast recovery of uranium from seawater by Bacillus velezensis strain UUS-1 with innate anti-biofouling activity. Adv. Sci. 6, 1900961 (2019).
Brogden, K. A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3, 238–250 (2005).
Sapra, R. et al. Designer peptide and protein dendrimers: a cross-sectional analysis. Chem. Rev. 119, 11391–11441 (2019).
Sader, H. S., Fedler, K. A., Rennie, R. P., Stevens, S. & Jones, R. N. Omiganan pentahydrochloride (MBI 226), a topical 12-amino-acid cationic peptide: spectrum of antimicrobial activity and measurements of bactericidal activity. Antimicrob. Agents Chemother. 48, 3112–3118 (2004).
Melo, M. N. & Castanho, M. A. R. B. Omiganan interaction with bacterial membranes and cell wall models. Assigning a biological role to saturation. Biochim. Biophys. Acta 1768, 1277–1290 (2007).
Rubinchik, E., Dugourd, D., Algara, T., Pasetka, C. & Friedland, H. D. Antimicrobial and antifungal activities of a novel cationic antimicrobial peptide, omiganan, in experimental skin colonisation models. Int. J. Antimicrob. Agents 34, 457–461 (2009).
Rozek, A., Powers, J. P., Friedrich, C. L. & Hancock, R. E. Structure-based design of an indolicidin peptide analogue with increased protease stability. Biochemistry 42, 14130–14138 (2003).
Wang, X. et al. Melamine modified graphene hydrogels for the removal of uranium(VI) from aqueous solution. New J. Chem. 41, 10899–10907 (2017).
Yuan, Y. H. et al. Ultrafast and highly selective uranium extraction from seawater by hydrogel-like spidroin-based protein fiber. Angew. Chem. Int. Ed. Engl. 58, 11785–11790 (2019).
Yuan, Y. H. et al. DNA nano-pocket for ultra-selective uranyl extraction from seawater. Nat. Commun. 11, 5708 (2020).
Ma, C. et al. Sunlight polymerization of poly(amidoxime) hydrogel membrane for enhanced uranium extraction from seawater. Adv. Sci. 6, 1900085 (2019).
Das, S. et al. Extracting uranium from seawater: promising AF series adsorbents. Ind. Eng. Chem. Res. 55, 4110–4117 (2016).
Abney, C. W. et al. XAFS investigation of polyamidoxime-bound uranyl contests the paradigm from small molecule studies. Energy Environ. Sci. 9, 448–453 (2016).
Schneider, E. & Sachde, D. The cost of recovering uranium from seawater by a braided polymer adsorbent system. Sci. Glob. Secur. 21, 134–163 (2013).
Pawlas, J. et al. Sustainable, cost-efficient manufacturing of therapeutic peptides using chemo-enzymatic peptide synthesis (CEPS). Green Chem. 21, 6451–6467 (2019).
Kim, J. et al. Uptake of uranium from seawater by amidoxime-based polymeric adsorbent: field experiments, modeling, and updated economic assessment. Ind. Eng. Chem. Res. 53, 6076–6083 (2014).
Lindner, H. & Schneider, E. Review of cost estimates for uranium recovery from seawater. Energy Econ. 49, 9–22 (2015).
Uranium Price (Cameco, 2020); https://www.cameco.com/invest/markets/uranium-price
Acknowledgements
This work was supported by the Hainan Science and Technology Major Project (ZDKJ2019013 and ZDKJ2020011), the National Natural Science Foundation of China (41966009, U1967213, 51775152 and 61761016), the Hainan Provincial Natural Science Foundation of China (2019CXTD401) and the National Key R&D programme of China (2018YFE0103500).
Author information
Authors and Affiliations
Contributions
Y.Y., Q.Y. and N.W. conceived the research and designed the experiments. Q.Y., M.C., L.F., S.F., T.L., T.F. and B.Y. carried out the experiment. All authors analysed the data. Y.Y., N.W. and Z.G. contributed to the project discussions. Y.Y., Q.Y. and N.W. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Sustainability thanks Shengqian Ma, Shuao Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–22, Tables 1–7, and references.
Rights and permissions
About this article
Cite this article
Yuan, Y., Yu, Q., Cao, M. et al. Selective extraction of uranium from seawater with biofouling-resistant polymeric peptide. Nat Sustain 4, 708–714 (2021). https://doi.org/10.1038/s41893-021-00709-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-021-00709-3
This article is cited by
-
Ultra-selective uranium separation by in-situ formation of π-f conjugated 2D uranium-organic framework
Nature Communications (2024)
-
Microbial-driven ectopic uranium extraction with net electrical energy production
Frontiers of Environmental Science & Engineering (2024)
-
Spontaneous electrochemical uranium extraction from wastewater with net electrical energy production
Nature Water (2023)
-
Ultrasensitive and highly selective detection of strontium ions
Nature Sustainability (2023)
-
Tuning excited state electronic structure and charge transport in covalent organic frameworks for enhanced photocatalytic performance
Nature Communications (2023)